A research team led by Professor Jaehoon Kim, Department of Mechanical Engineering,
Revealed the Carrier-Ion Storage Mechanisms of Electrode Materials
in Large Capacity Low Cost Carbon Batteries
-Revealed the ionstorage mechanisms of lithium, sodium, and potassium in hard carbon cathodematerials
-Selected as coverarticle of Advanced Energy Materials, a world-renowned journal
 [Image1]From left to right, Professor Jaehoon Kim, Department of Mechanical Engineering,
[Image1]From left to right, Professor Jaehoon Kim, Department of Mechanical Engineering,
ProfessorSang Kyu Kwak, Ulsan National Institute of Science and Technology,
Researcher Handi Setiadi Cahyadi, SKKU Advanced Institute of Nano Technology(SAINT)
A research team led by Professor Jaehoon Kim, Department of Mechanical Engineering, College of Engineering, Sungkyunkwan University, conducted joint research with a research team led by Professor Sang Kyu Kwak, Ulsan National Institute of Science and Technology. The co-first authors of the research article are researchers Stevanus Alvin and Handi Setiadi Cahyadi. The joint research team revealed the ion storage mechanisms of lithium, sodium, and potassium in hard carbon, which is the most promising anode material that can be used by mid-large size batteries to store energy. The research presented a new path towards developing safe and high-capacity materials.
In order to continuously utilize renewable energy sources that do not have stabilized electric power output, such as solar and wind power, it is essential to develop a medium-large size energy storage system that can store renewable electricity and use it when necessary. However, with lithium-ion batteries, it is difficult to develop energy storage systems due to their instability and high prices. Therefore, batteries that store the very abundant and low-priced elements of sodium and potassium in hard carbon are currently in the spotlight, but the ion storage mechanisms had not been established, making it hard to develop high-capacity batteries.
The research team synthesized hard carbon prepared by the carbonization of lignin and conducted a systematic study on the changes in the physicochemical properties of hard carbon that change during the charging and discharging of lithium, sodium, and potassium. Furthermore, using the density functional theory, two facts were revealed: 1. The graphene layers were expanded during the sodium and potassium insertion into hard carbon, and 2. There is a larger capacity in sodium and potassium than lithium because there was no expansion.
This study is expected to provide ways to improve safe and large capacity medium-large sized power storage batteries and play an important role in the development of new and renewable energy power storage systems.
Researcher Handi Setiadi Cahyadi said, “Through this research, the ion storage mechanism in hard carbon, which has been a topic of debate, has been made clear, so now low cost anode materials can be developed to facilitate the creation of mid-large sized power storage.”
This research was supported by the Technology Development Program to Solve Climate Changes of the National Research Foundation (NRF) funded by the Ministry of Science, ICT & Future Planning (2017M1A2A2087635). Additional support from the Waste‐to‐Energy Technology Development Program of the Korea Environmental Industry & Technology Institute with financial resources provided by the Ministry of Environment, Republic of Korea (No. 2018001580001) was also appreciated.
The research article was published in Advanced Energy Materials, a world-renowned journal in the field of materials science, on April 15th (Wed). Also, it was selected as the cover article of the journal on May 26th (Tue).
※ Original Article: Intercalation Mechanisms: Revealing the Intercalation Mechanisms of Lithium, Sodium, and Potassium in Hard Carbon
※ Source: https://onlinelibrary.wiley.com/doi/abs/10.1002/aenm.202000283
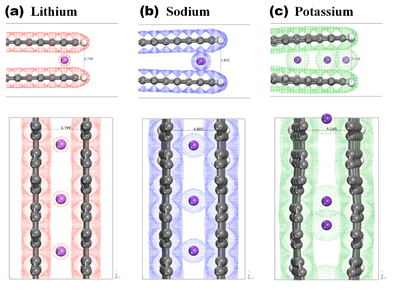
[Image2]
The Schematic diagrams showing the changes in the ions, ionic radius, and electric potential in the carbon layer during the Li, Na, K- atom insertions into the hard carbon
The K- ions hadthe largest ionic radius and revealed the largest edge expansion, making successiveK-ions easily penetrate deeper into hard carbon vacancies between layers.